-
Photophysical properties of three-dimensional transition metal tris-oxalate network structures
A. Hauser, M.E. Von Arx, V.S. Langford, S. Kairouani, U. Oetliker and A. Pillonnet
in "Topics in Current Chemistry, Transition Metal and Rare Earth Compounds. Excited States, Transitions, and Interactions, Vol III" (ed. H. Yersin), Springer, Berlin, 241 (2004)


DOI:10.1007/b96860 | unige:3941
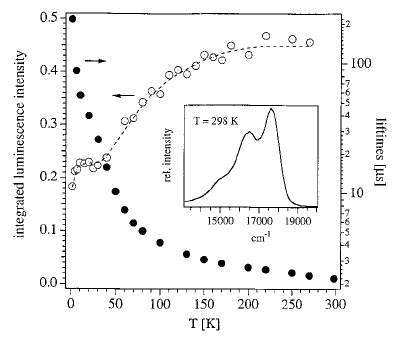
Excitation energy transfer processes play an important role in many areas of physics, chemistry and biology. The three-dimensional oxalate networks of composition [MIII(bpy)3][MIMIII(ox)3]ClO4 (bpy=2,2-bipyridine, ox=oxalate, MI=alkali ion) allow for a variety of combinations of different transition metal ions. The combination with chromium(III) on both the tris-bipyridine as well as the tris-oxalate site constitutes a model system in which it is possible to differentiate unambiguously between energy transfer from [Cr(ox)3]3‚Äď to [Cr(bpy)3]3+ due to dipole-dipole interaction on the one hand and exchange interaction on the other hand. Furthermore it is possible to just as unambiguously differentiate between the common temperature dependent phonon-assisted energy migration within the 2E state of [Cr(ox)3]3‚Äď, and a unique resonant process.